Calcium Carbonate Reacts With Hydrochloric Acid
Calcium phosphate appears as a white amorphous or crystalline powder that is odourless and tasteless. It is insoluble in ethanol and acetic acid but soluble in dilute nitric acid and hydrochloric acid.
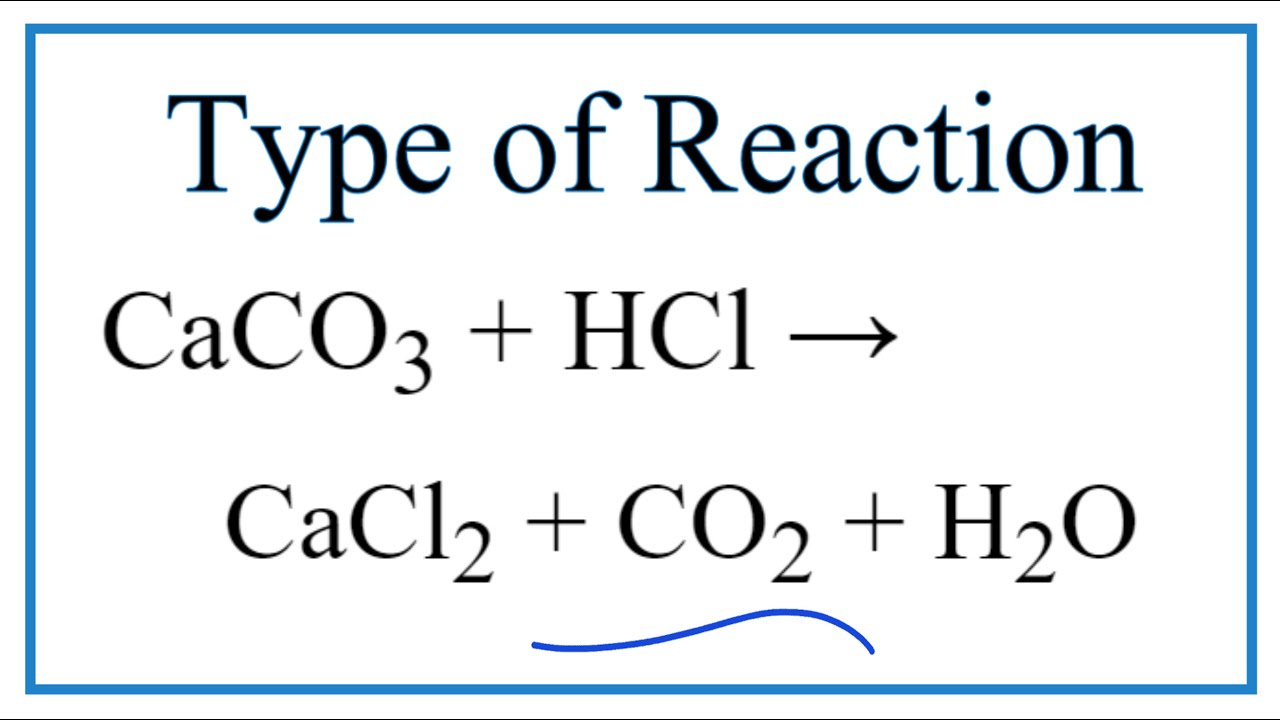
Type Of Reaction For Caco3 Hcl Cacl2 Co2 H2o Youtube
You get immediate fizzing with a colourless gas given off - thats carbon dioxide.
. HClaq H aq Cl aq A strong base is one that is fully dissociated in aqueous solution. Similarly when sulphuric acid reacts with zinc oxide zinc sulphate and water are formed. The acid is seen to be donating two protons as the sulfate ion is seen to acquire negative charges.
Metal compound A reacts with dilute hydrochloric acid to produce effervescence. If acidified barium chloride is added to a solution that contains sulfate ions a white precipitate of barium sulfate forms. Since calcium carbonate is relatively insoluble it tends to come out of solution.
CaCO 3 CaO. The hydroxide ions from the magnesium hydroxide suspension will combine with the acidic H ions of the hydrochloric acid made by the stomachs parietal cells. So metal compound A is a carbonate of calcium which on reacting with HCl gives calcium chloride and carbon dioxide as gas along with water.
When it reacts with dilute acid it liberates carbon dioxide as a by-product. 240 cm 3 of carbon dioxide gas is collected. In general a neutralisation reaction can be written as Base Acid Salt W ater 215 Reaction of Metallic Oxides with Acids Activity 27 n Take a small amount of copper oxide in a beaker and add dilute hydrochloric acid slowly.
Testing for Presence of a Sulfate ion BaCl2 solution acidified with hydrochloric acid is used as a reagent to test for sulphate ions. The marble reacts to give. In the case of hydrochloric acid HCl HCl H Cl-The number of hydrogen ions or hydronium ions released by hydrochloric acid is one.
NaOHaq Na aq OH aq Therefore when a strong acid reacts with a strong base the neutralization reaction can be. 01 g of calcium carbonate is added to excess hydrochloric acid. Zinc reacts with dilute hydrochloric acid to produce zinc.
The reaction stops after 15 seconds. Calcium carbonate is made up of 28 grams of calcium oxide and 22 grams of carbon dioxide. When a metal compound A on reacting with hydrochloric acid shows effervescence which shows the evolution of carbon dioxide gas and it is confirmed by putting off the candle flame.
Beginarraylendarray CaCO 3 H 2 SO 4 CaSO 4 H 2 OCO 2. The commonest carbonate-acid reaction you will come across is that between calcium carbonate and dilute hydrochloric acid. Write a balanced chemical equation for the reaction if one of the compounds formed is calcium chloride.
It is found in bones milk teeth and ground. Calcium is a metal thus calcium oxide is a metallic oxide which is basic in nature. BaSO4 is the least soluble.
Calcium carbonate occurs naturally as chalk limestone and marble. This neutralization reaction will result in the formation. So the valency factor will be one.
Limestone is a common type of carbonate sedimentary rock which is the main source of the material limeIt is composed mostly of the minerals calcite and aragonite which are different crystal forms of calcium carbonate CaCO 3Limestone forms when these minerals precipitate out of water containing dissolved calcium. For example hydrochloric acid HCl is a strong acid. Answer CaCO 3 s 2HCl aq CaCl 2 aq CO 2 g H 2 O l.
For example sodium hydroxide NaOH is a strong base. The reaction between an acid and a base to give a salt and water is known as a neutralisation reaction. Therefore the equivalent weight of the acid would be.
As it reacts with the hydrochloric acid it forms soluble calcium chloride. When an acid such as hydrochloric acid reacts with calcium oxide neutralization reaction takes place and calcium chloride along with water is formed. Calculate the average rate of reaction in a g s-1 b mol s-1 c cm 3 s-1.
This can take place through both biological and. Calcium carbonate also called limestone is an example of a metal carbonate used in the Solvay process. 3 Group II sulfates become less soluble down the group.
The calcium carbonate is a white powder that mixes with water but does not dissolve. The photo shows the reaction with marble chips. Sodium carbonate partially breaks down at high temperature to sodium hydroxide caustic and carbon dioxide.
Calcium bicarbonate entering with the feed water is broken down at boiler temperatures or reacts with caustic soda to form calcium carbonate. The gas evolved extinguishes a burning candle. It slightly dissolves in water.
Similarly when calcium carbonate reacts with hydrochloric acid increasing the concentration of the acid speeds up the rate of reaction as long as enough calcium carbonate is present. At 1200K calcium carbonate decomposes to give carbon dioxide and calcium oxide. Through the ingestion of 05-15 grams in adults the magnesium hydroxide will act by simple acid neutralization in the stomach.
What Is The Reaction Between Calcium Carbonate And Hydrochloric Acid Quora

Caco3 Hcl Calcium Carbonate Hydrochloric Acid Youtube

How To Balance Caco3 Hcl Cacl2 Co2 H2o Calcium Carbonate Hydrochloric Acid Youtube

How To Balance Caco3 Hcl Cacl2 Co2 H2o Calcium Carbonate Hydrochloric Acid Youtube
No comments for "Calcium Carbonate Reacts With Hydrochloric Acid"
Post a Comment